Chemistry Department. The Islamic University of Gaza. General Chemistry B.(CHEMB 1301) Time:2 hours الرقم الجامعي... اسم المدرس...
|
|
|
- Kevin Hubbard
- 5 years ago
- Views:
Transcription
1 The Islmic University of Gz Chemistry Deprtment Generl Chemistry B.(CHEMB 1301) Time:2 hours 60 اسم الطالب... الرقم الجامعي... اسم المدرس... R = J/mol.K, or = L.tm/mol.K Q1- True ( ) or flse( X ) 1- ( ) The term use to escribe resistnce to flow of liqui is surfce tension. F 2- ( ) The entropy chnge on vporiztion (ΔS vp ) of compoun or element is lwys negtive. F 3- ( ) A rection is spontneous t ll tempertures if ΔH is negtive n ΔS is positive. T 4- ( ) In n electrolytic cell, electricl energy is converte into chemicl energy. T 5- ( ) The rte constnt of first-orer rection cn be etermine from grph of ln [A] versus t. T 6- ( ) The freezing point of the solution is lwys higher thn the freezing point of the pure solvent. F 7-( ) The solubility of gses in wter lwys ecreses with incresing temperture. T 8-( ) For the rection H 2 (g) + I 2 (g) 2HI(g), K p n K c hve the sme vlue. T 9-( ) In the rection, 2N 2 O 2N 2 + O 2, oxygen gs is forme t the sme rte s nitrogen gs. F 10-( ) For ny conjugte ci-bse pir, it is true tht K b = K K w. F Q2- Choose the correct nswer: No Answer No Answer 1
2 1- Blnce the following eqution in ciic solution n etermine the coefficient of H + n its loction (right or left sie) in the eqution. I 2 + ClO 4 IO 3 + ClO 3. 4, right b. 2, right c. 6, right. 4, left 2- Consier the following rection: Cu 2+ (q) + Zn Zn 2+ (q) + Cu Clculte emf ( E )of the cell if [Zn 2+ ] = 0.25 M n [Cu 2+ ] = 0.15 M Given: E o Zn 2+ /Zn = V, E o Cu 2+ /Cu = 0.34 V V b V c V. 0 V 3- A current of 0.80 A ws pplie to n electrolytic cell contining molten CCl 2 for 2.5 hours. Clculte the mss of cmium Metl (C = 112.4) eposite. 1F = 96,500 C g b. 8.4 g c. 4.2 g g 4- An electrochemicl cell bse on the following rection hs stnr cell voltge (E o cell) of 0.48 V. Sn(s) + Cu 2+ (q) Sn 2+ (q) + Cu(s) Given: E o Cu 2+ /Cu = V, wht is the E o Sn 2+ /Sn V b V c V V 5- Arrnge the following rections ccoring to incresing ΔS. 1. CH 4 (g) + H 2 O(g) CO(g) + 3H 2 (g) 2. C(s) + O 2 (g) CO 2 (g) 3. H 2 O 2 (l) H 2 O(l) + 1/2O 2 (g). 1 < 3 < 2 b. 2 < 3 < 1 c. 2 < 1 < 3. 3 < 2 < 1 2
3 6- The equilibrium constnt t 427 o C for the rection N 2 (g) + 3H 2 (g) 2NH 3 (g) is K p = Clculte the vlue of ΔG o for the rection t 427 o C.. 54 kj b. 54 kj c. 33 kj. 33 kj 7- Which of the following sttements bout ΔG is FALSE?. spontneous rections lwys hve negtive ΔG vlues b. ΔG is t stnr conitions (1 tm, 1 M) c. ΔG = ΔH TΔS. spontneous rections re lwys exothermic 8- Consier the following rection: 2NO(g) + N 2 (g) 2N 2 O(g) ΔH o = 16 kj/mol Given: Sº(NO) = 210 J/molK, Sº(N 2 ) = 190 J/molK, Sº(N 2 O) = 220 J/molK. At wht temperture will ΔGº = 0 n below which the rection will be spontneous?. 3.9 K c. 321 K. 94 K. 367K 9- Consier the rection: Mg(OH) 2 (s) Mg 2+ (q) + 2OH (q) Ksp = Wht is the ph of the solution?. 3.7 b c Wht is the ph of solution in which 25.0 ml of M NOH re e to 10.0 ml of M HCl? b c
4 11- Which of the following slts will be the lest soluble in H 2 O?. AgCl (Ksp = 1 x 10 8 ) b. AgF (Ksp = 1 x 10 6) c. AgI (Ksp = 1 x ). AgBr (Ksp = 1 x ) 12- Wht is the ph of 0.01 M solution of KOCl. k (HOCl) = 1 x b. 4.0 c Wht is the ph of 0.1 M solution of bse tht hs K b = 1 x b. 3.0 c Which of the following slts will prouce bsic solution when issolve in wter?. KClO 4 b. NH 4 NO 3 c. Li 2 CO 3. N 2 SO Consier the rection: H 2 (g) + H 2 N-NH 2 (g) 2NH 3 (g) Keq = 36 Initilly, [H 2 ] = [H 2 N-NH 2 ] = 0.2 M, n [NH 3 ] = 2 M. Wht is the concentrtion of [NH 3 ] t equilibrium?. 2.2 b. 0.2 c Wht is the equilibrium constnt for the following rection: 2NO(g) + Cl 2 (g) 2NOCl(g) when [NO] = 4M, [Cl 2 ] = 2M, n [NOCl] = 2M t equilibrium b. 2 c
5 17- Consier the following rection: CCO 3 (s) + 2HCl(q) CO 2 (q) + CCl 2 (q) + H 2 O(l) ΔHºrxn = 50 kj/mol. Which of the following will NOT shift the equilibrium to mke more proucts?. Aing more HCl(q) b. Aing more CCO 3 (s) c. Removing CCl 2 (q). Cooling the rection 18- Consier the following rections. In which cses is the prouct formtion fvore by ecrese temperture? 1) CO 2 (g) + C(s) 2 CO(g) H o = kj 2) N 2 (g) + 3 H 2 (g) 2 NH 3 (g) H o = kj 3) CO(g) + 2H 2 (g) CH 3 OH H o = kj 4) N 2 (g) + O 2 (g) 2 NO(g) H o = 181 kj. 2, 3 b. 1, 4 c. 2, 4. 3, Wht is the kinetic rte lw for the following t: Exp # [A] [B] Rte rte = k[a][b] b. rte = k[a] 2 [B] c. rte = k[a][b] 2. rte = k[a] 20- The enthlpy of ctivtion of first orer rection is 105 kj/mole. The rte constnt t 45 o C is 3.54 x 10-5 s -1. Wht is the rte constnt (s -1 ) t 60 o C? x 10-5 b x 10-5 c x x The rte constnt of secon orer rection is 3.83 x 10-3 M -1 s -1. Wht concentrtion of rectnt will remin fter 142 sec for n initil concentrtion of M? b c
6 g of non electrolytic solute is issolve in 20 g of liqui to elevte the boiling point of the liqui by C. Wht is the moleculr weight of solute? K b = o C/m g/mol b g/mol c. 202 g/mol. 234 g/mol 23- Which of the following queous solutions shoul hve the lowest osmotic pressure? M sucrose t 25 o C b M NCl t 25 o C c M CCl 2 t 25 o C M sucrose t 50 o C 24- The ensity of M solution of Pb(NO 3 ) 2 solution is 1.18 g/cm 3? Wht is the mollity of the solution? (formul weights of Pb(NO 3 ) 2 = 331.2) b c Which chrcterizes the wek inter moleculr forces of ttrction in liqui?. High boiling point b. High vpour pressure c. High criticl temperture. High het of vporiztion Goo Luck 6
Homework 04. Acids, Bases, and Salts
 HW04 - Acids, Bses, nd Slts! This is preview of the published version of the quiz Strted: Feb 21 t 8:59m Quiz Instruc!ons Homework 04 Acids, Bses, nd Slts Question 1 In the reversible rection HCN + H O
HW04 - Acids, Bses, nd Slts! This is preview of the published version of the quiz Strted: Feb 21 t 8:59m Quiz Instruc!ons Homework 04 Acids, Bses, nd Slts Question 1 In the reversible rection HCN + H O
Which of the following describes the net ionic reaction for the hydrolysis. Which of the following salts will produce a solution with the highest ph?
 95. Which of the following descries the net ionic rection for the hydrolysis of NH4Cl( s)? A. NH4 ( q) Cl & ( q) NH4Cl( s) B. NH Cl & 4 ( s) NH4 ( q) Cl ( q) C. Cl ( q) H O & 2 ( l) HCl( q) OH ( q) D.
95. Which of the following descries the net ionic rection for the hydrolysis of NH4Cl( s)? A. NH4 ( q) Cl & ( q) NH4Cl( s) B. NH Cl & 4 ( s) NH4 ( q) Cl ( q) C. Cl ( q) H O & 2 ( l) HCl( q) OH ( q) D.
Cu 3 (PO 4 ) 2 (s) 3 Cu 2+ (aq) + 2 PO 4 3- (aq) circle answer: pure water or Na 3 PO 4 solution This is the common-ion effect.
 CHEM 1122011 NAME: ANSWER KEY Vining, Exm # SHORT ANSWER QUESTIONS ============= 4 points ech ============ All work must be shown. 1. Wht re [H O ] nd [OH ] for solution tht hs ph of 9.0? Choose one. ()
CHEM 1122011 NAME: ANSWER KEY Vining, Exm # SHORT ANSWER QUESTIONS ============= 4 points ech ============ All work must be shown. 1. Wht re [H O ] nd [OH ] for solution tht hs ph of 9.0? Choose one. ()
7/19/2011. Models of Solution Chemistry- III Acids and Bases
 Models of Solution Chemistry- III Acids nd Bses Ionic Atmosphere Model : Revisiting Ionic Strength Ionic strength - mesure of totl concentrtion of ions in the solution Chpter 8 1 2 i μ ( ) 2 c i z c concentrtion
Models of Solution Chemistry- III Acids nd Bses Ionic Atmosphere Model : Revisiting Ionic Strength Ionic strength - mesure of totl concentrtion of ions in the solution Chpter 8 1 2 i μ ( ) 2 c i z c concentrtion
Chapter 17: Additional Aspects of Aqueous Equilibria
 1 Chpter 17: Additionl Aspects of Aqueous Equilibri Khoot! 1. Adding Br to sturted queous solution of decreses its solubility in wter. BSO 4, Li CO 3, PbS, AgBr. Which of the following mitures could be
1 Chpter 17: Additionl Aspects of Aqueous Equilibri Khoot! 1. Adding Br to sturted queous solution of decreses its solubility in wter. BSO 4, Li CO 3, PbS, AgBr. Which of the following mitures could be
Dynamic equilibrium occurs when the forward and reverse reactions occur at the same rate.
 MODULE 2 WORKSHEET8 EQUILIBRIUM Syllus reference 9.3.2 1 Clssify ech of the following sttements s true or flse. For the flse sttements rewrite them so they re true. For chemicl equilirium to e estlished
MODULE 2 WORKSHEET8 EQUILIBRIUM Syllus reference 9.3.2 1 Clssify ech of the following sttements s true or flse. For the flse sttements rewrite them so they re true. For chemicl equilirium to e estlished
CHAPTER 08: MONOPROTIC ACID-BASE EQUILIBRIA
 Hrris: Quntittive Chemicl Anlysis, Eight Edition CHAPTER 08: MONOPROTIC ACIDBASE EQUILIBRIA CHAPTER 08: Opener A CHAPTER 08: Opener B CHAPTER 08: Opener C CHAPTER 08: Opener D CHAPTER 08: Opener E Chpter
Hrris: Quntittive Chemicl Anlysis, Eight Edition CHAPTER 08: MONOPROTIC ACIDBASE EQUILIBRIA CHAPTER 08: Opener A CHAPTER 08: Opener B CHAPTER 08: Opener C CHAPTER 08: Opener D CHAPTER 08: Opener E Chpter
Hydronium or hydroxide ions can also be produced by a reaction of certain substances with water:
 Chpter 14 1 ACIDS/BASES Acids hve tste, rect with most metls to produce, rect with most crbontes to produce, turn litmus nd phenolphthlein. Bses hve tste rect very well well with most metls or crbontes,
Chpter 14 1 ACIDS/BASES Acids hve tste, rect with most metls to produce, rect with most crbontes to produce, turn litmus nd phenolphthlein. Bses hve tste rect very well well with most metls or crbontes,
Ch. 24 Molecular Reaction Dynamics 1. Collision Theory 2. Diffusion-Controlled Reaction
 Ch. 4 Moleculr Rection Dynmics 1. Collision Theory. Diffusion-Controlle Rection Lecture 17 3. The Mteril Blnce Eqution 4. Trnsition Stte Theory: The Eyring Eqution 5. Trnsition Stte Theory: Thermoynmic
Ch. 4 Moleculr Rection Dynmics 1. Collision Theory. Diffusion-Controlle Rection Lecture 17 3. The Mteril Blnce Eqution 4. Trnsition Stte Theory: The Eyring Eqution 5. Trnsition Stte Theory: Thermoynmic
Acids and Bases. H + (aq) + Cl - (aq) 100 molecules HCl 100 H+ ions Cl- ions 100% HCl molecules dissociate in water.
 Acids nd Bses Acids: in generl cid is the substnce tht produces H ions when it is dissolved in wter. Acids cn be divided into two different clsses: Strong cid: is one tht completely dissocites into its
Acids nd Bses Acids: in generl cid is the substnce tht produces H ions when it is dissolved in wter. Acids cn be divided into two different clsses: Strong cid: is one tht completely dissocites into its
CHEMISTRY 16 HOUR EXAM III March 26, 1998 Dr. Finklea - Answer key
 CHEMISTRY 16 HOUR EXAM III Mrch 26, 1998 Dr. Finkle - Answer key 1. Phenol, lso known s crbolic cid, is wek monoprotic cid (HA). A 1.0 M solution of phenol hs ph of 4.95. Wht is the K of phenol? 1. 2.0
CHEMISTRY 16 HOUR EXAM III Mrch 26, 1998 Dr. Finkle - Answer key 1. Phenol, lso known s crbolic cid, is wek monoprotic cid (HA). A 1.0 M solution of phenol hs ph of 4.95. Wht is the K of phenol? 1. 2.0
Fundamentals of Analytical Chemistry
 Homework Fundmentls of nlyticl hemistry hpter 9 0, 1, 5, 7, 9 cids, Bses, nd hpter 9(b) Definitions cid Releses H ions in wter (rrhenius) Proton donor (Bronsted( Lowry) Electron-pir cceptor (Lewis) hrcteristic
Homework Fundmentls of nlyticl hemistry hpter 9 0, 1, 5, 7, 9 cids, Bses, nd hpter 9(b) Definitions cid Releses H ions in wter (rrhenius) Proton donor (Bronsted( Lowry) Electron-pir cceptor (Lewis) hrcteristic
1. Weak acids. For a weak acid HA, there is less than 100% dissociation to ions. The B-L equilibrium is:
 th 9 Homework: Reding, M&F, ch. 15, pp. 584-598, 602-605 (clcultions of ph, etc., for wek cids, wek bses, polyprotic cids, nd slts; fctors ffecting cid strength). Problems: Nkon, ch. 18, #1-10, 16-18,
th 9 Homework: Reding, M&F, ch. 15, pp. 584-598, 602-605 (clcultions of ph, etc., for wek cids, wek bses, polyprotic cids, nd slts; fctors ffecting cid strength). Problems: Nkon, ch. 18, #1-10, 16-18,
APEF Acids and Bases - Answers
 APEF Acids nd Bses - Answers 1. It requires 3.0 ml of 0.0500 mol/l NOH(q) to neutrlize 100.0 ml of gstric juice. We cn ssume tht HCl(q) is the only cid present in gstric juice. ) Clculte the concentrtion
APEF Acids nd Bses - Answers 1. It requires 3.0 ml of 0.0500 mol/l NOH(q) to neutrlize 100.0 ml of gstric juice. We cn ssume tht HCl(q) is the only cid present in gstric juice. ) Clculte the concentrtion
CHEMICAL KINETICS
 CHEMICAL KINETICS Long Answer Questions: 1. Explin the following terms with suitble exmples ) Averge rte of Rection b) Slow nd Fst Rections c) Order of Rection d) Moleculrity of Rection e) Activtion Energy
CHEMICAL KINETICS Long Answer Questions: 1. Explin the following terms with suitble exmples ) Averge rte of Rection b) Slow nd Fst Rections c) Order of Rection d) Moleculrity of Rection e) Activtion Energy
Module 2: Rate Law & Stoichiomtery (Chapter 3, Fogler)
 CHE 309: Chemicl Rection Engineering Lecture-8 Module 2: Rte Lw & Stoichiomtery (Chpter 3, Fogler) Topics to be covered in tody s lecture Thermodynmics nd Kinetics Rection rtes for reversible rections
CHE 309: Chemicl Rection Engineering Lecture-8 Module 2: Rte Lw & Stoichiomtery (Chpter 3, Fogler) Topics to be covered in tody s lecture Thermodynmics nd Kinetics Rection rtes for reversible rections
Dr. Steward s CHM152 Exam #2 Review Spring 2014 (Ch ) KEY
 Dr. Stewrd s CHM152 Em #2 Review Spring 2014 (Ch. 16. 17) KEY 1. Eplin the commonion effect. Wek cid or wek bse s % dissocition will decrese if they re plced in solutions with one of the products of dissocition.
Dr. Stewrd s CHM152 Em #2 Review Spring 2014 (Ch. 16. 17) KEY 1. Eplin the commonion effect. Wek cid or wek bse s % dissocition will decrese if they re plced in solutions with one of the products of dissocition.
4. CHEMICAL KINETICS
 4. CHEMICAL KINETICS Synopsis: The study of rtes of chemicl rections mechnisms nd fctors ffecting rtes of rections is clled chemicl kinetics. Spontneous chemicl rection mens, the rection which occurs on
4. CHEMICAL KINETICS Synopsis: The study of rtes of chemicl rections mechnisms nd fctors ffecting rtes of rections is clled chemicl kinetics. Spontneous chemicl rection mens, the rection which occurs on
Rates of chemical reactions
 Rtes of chemicl rections Mesuring rtes of chemicl rections Experimentl mesuring progress of the rection Monitoring pressure in the rection involving gses 2 NO( g) 4 NO ( g) + O ( g) 2 5 2 2 n(1 α) 2αn
Rtes of chemicl rections Mesuring rtes of chemicl rections Experimentl mesuring progress of the rection Monitoring pressure in the rection involving gses 2 NO( g) 4 NO ( g) + O ( g) 2 5 2 2 n(1 α) 2αn
Chapter 15. Applications of Aqueous Equlibria. A bag of mostly water - Star Trek -
 Chpter 15. Applictions of Aqueous Equlibri A bg of mostly wter - Str Trek - Solutions of Acids or Bses Contining Common Ion Until now, we were concerned with the equilibrium of solution with n cid or bse.
Chpter 15. Applictions of Aqueous Equlibri A bg of mostly wter - Str Trek - Solutions of Acids or Bses Contining Common Ion Until now, we were concerned with the equilibrium of solution with n cid or bse.
9-1 (a) A weak electrolyte only partially ionizes when dissolved in water. NaHCO 3 is an
 Chpter 9 9- ( A ek electrolyte only prtilly ionizes hen dissolved in ter. NC is n exmple of ek electrolyte. (b A Brønsted-ory cid is cule tht dontes proton hen it encounters bse (proton cceptor. By this
Chpter 9 9- ( A ek electrolyte only prtilly ionizes hen dissolved in ter. NC is n exmple of ek electrolyte. (b A Brønsted-ory cid is cule tht dontes proton hen it encounters bse (proton cceptor. By this
CHE 107 Spring 2018 Exam 2
 CHE 107 Spring 2018 Exam 2 Your Name: Your ID: Question #: 1 Which substance has the smallest standard molar entropy ( S)? A He(g) B H2O(g) C CH4(g) D F2(g) Question #: 2 Phosgene, a chemical weapon used
CHE 107 Spring 2018 Exam 2 Your Name: Your ID: Question #: 1 Which substance has the smallest standard molar entropy ( S)? A He(g) B H2O(g) C CH4(g) D F2(g) Question #: 2 Phosgene, a chemical weapon used
Chapter 16 Acid Base Equilibria
 Chpter 16 Acid Bse Equilibri 16.1 Acids & Bses: A Brief Review Arrhenius cids nd bses: cid: n H + donor HA(q) H(q) A(q) bse: n OH donor OH(q) (q) OH(q) Brønsted Lowry cids nd bses: cid: n H + donor HA(q)
Chpter 16 Acid Bse Equilibri 16.1 Acids & Bses: A Brief Review Arrhenius cids nd bses: cid: n H + donor HA(q) H(q) A(q) bse: n OH donor OH(q) (q) OH(q) Brønsted Lowry cids nd bses: cid: n H + donor HA(q)
UNIVERSITY OF MALTA DEPARTMENT OF CHEMISTRY. CH237 - Chemical Thermodynamics and Kinetics. Tutorial Sheet VIII
 UNIVERSITY OF MALTA DEPARTMENT OF CHEMISTRY CH237 - Chemicl Thermodynmics nd Kinetics Tutoril Sheet VIII 1 () (i) The rte of the rection A + 2B 3C + D ws reported s 1.0 mol L -1 s -1. Stte the rtes of
UNIVERSITY OF MALTA DEPARTMENT OF CHEMISTRY CH237 - Chemicl Thermodynmics nd Kinetics Tutoril Sheet VIII 1 () (i) The rte of the rection A + 2B 3C + D ws reported s 1.0 mol L -1 s -1. Stte the rtes of
APPENDIX. Precalculus Review D.1. Real Numbers and the Real Number Line
 APPENDIX D Preclculus Review APPENDIX D.1 Rel Numers n the Rel Numer Line Rel Numers n the Rel Numer Line Orer n Inequlities Asolute Vlue n Distnce Rel Numers n the Rel Numer Line Rel numers cn e represente
APPENDIX D Preclculus Review APPENDIX D.1 Rel Numers n the Rel Numer Line Rel Numers n the Rel Numer Line Orer n Inequlities Asolute Vlue n Distnce Rel Numers n the Rel Numer Line Rel numers cn e represente
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this mteril useful? You cn help our tem to keep this site up nd bring you even more content consider donting vi the link on our site. Still hving trouble understnding the mteril? Check out our Tutoring
Find this mteril useful? You cn help our tem to keep this site up nd bring you even more content consider donting vi the link on our site. Still hving trouble understnding the mteril? Check out our Tutoring
3. The osmotic pressure of a ml solution of an unknown nonelectrolyte is 122 torr at 25 C. Determine the molarity of the solution.
 1. Which of the following has a correct van t Hoff factor indicated? A. Al 2 (SO 4 ) 3, i = 5 C. CaBr 2, i = 2 B. Na 2 CO 3, i = 6 D. C 6 H 12 O 6, i = 3 2. Calculate the vapor pressure of a solution containing
1. Which of the following has a correct van t Hoff factor indicated? A. Al 2 (SO 4 ) 3, i = 5 C. CaBr 2, i = 2 B. Na 2 CO 3, i = 6 D. C 6 H 12 O 6, i = 3 2. Calculate the vapor pressure of a solution containing
[HCO ] log = M. + log = log = log [CH
![[HCO ] log = M. + log = log = log [CH [HCO ] log = M. + log = log = log [CH](/thumbs/75/72569575.jpg) Chpter 17: 7, 9, 11, 1, 15, 1, 7,, 9, 4, 49, 50, 51, 79, 81, 8, 87, 9, 99, 105, 11, 117 [HCO ] 7. ph = pk + log [H CO ] [HCO ] [H CO ] log = 1.6 [HCO ] = 0.04 [HCO ] [NH ] 0.15 M 9. ph = pk + log = 9.5
Chpter 17: 7, 9, 11, 1, 15, 1, 7,, 9, 4, 49, 50, 51, 79, 81, 8, 87, 9, 99, 105, 11, 117 [HCO ] 7. ph = pk + log [H CO ] [HCO ] [H CO ] log = 1.6 [HCO ] = 0.04 [HCO ] [NH ] 0.15 M 9. ph = pk + log = 9.5
Category I (Q.41 to Q.70)
 CHEMISTRY Ctegory I (Q.41 to Q.70) Only one nswer is correct. Correct nswer will fetch full mrks 1. Incorrect nswer or ny combintion of more thn one nswer will fetch ¼ mrks. No nswer will fetch 0 mrks.
CHEMISTRY Ctegory I (Q.41 to Q.70) Only one nswer is correct. Correct nswer will fetch full mrks 1. Incorrect nswer or ny combintion of more thn one nswer will fetch ¼ mrks. No nswer will fetch 0 mrks.
GENERAL CHEMISTRY II CHM202 Unit 4 Practice Test
 GENERAL CHEMISTRY II CHM202 Unit 4 Practice Test This test is intended to help you get acquainted with the types of questions you will be asked on the Unit Test administered at the end of the unit. The
GENERAL CHEMISTRY II CHM202 Unit 4 Practice Test This test is intended to help you get acquainted with the types of questions you will be asked on the Unit Test administered at the end of the unit. The
Conservation Law. Chapter Goal. 6.2 Theory
 Chpter 6 Conservtion Lw 6.1 Gol Our long term gol is to unerstn how mthemticl moels re erive. Here, we will stuy how certin quntity chnges with time in given region (sptil omin). We then first erive the
Chpter 6 Conservtion Lw 6.1 Gol Our long term gol is to unerstn how mthemticl moels re erive. Here, we will stuy how certin quntity chnges with time in given region (sptil omin). We then first erive the
3.2.2 Kinetics. Maxwell Boltzmann distribution. 128 minutes. 128 marks. Page 1 of 12
 3.. Kinetics Mxwell Boltzmnn distribution 8 minutes 8 mrks Pge of M. () M On the energy xis E mp t the mximum of the originl pek M The limits for the horizontl position of E mp re defined s bove the word
3.. Kinetics Mxwell Boltzmnn distribution 8 minutes 8 mrks Pge of M. () M On the energy xis E mp t the mximum of the originl pek M The limits for the horizontl position of E mp re defined s bove the word
30. a. Al(H 2 O) 6 + H 2 O Co(H 2 O) 5 (OH) 2+ + H 3 O + acid base conjugate base conjugate acid
 Chpter 14 : xx,3,40,4,xx,xx,47,50,54,55,xx,64,xx,83,85,91,93,95,10,11,134,138 xx.. HC H 3 O (q) H O(l) C H 3 O (q) H 3 O (q) = [H O ][C H O ] 3 3 [HC H O ] 3 b. Co(H O) 6 3 (q) H O(l) Co(H O) 5 (OH) (q)
Chpter 14 : xx,3,40,4,xx,xx,47,50,54,55,xx,64,xx,83,85,91,93,95,10,11,134,138 xx.. HC H 3 O (q) H O(l) C H 3 O (q) H 3 O (q) = [H O ][C H O ] 3 3 [HC H O ] 3 b. Co(H O) 6 3 (q) H O(l) Co(H O) 5 (OH) (q)
2017. Riga Stradin s University
 2017. Rig Strin s University http://ris.gusc.lv/biothermoynmics/biohemiclpprocese.pf Thermoynmics et motion: Greek, Ltin lnguges U - Internl Energy; Enthlpy, et content; S Entropy, on chnges (entity):
2017. Rig Strin s University http://ris.gusc.lv/biothermoynmics/biohemiclpprocese.pf Thermoynmics et motion: Greek, Ltin lnguges U - Internl Energy; Enthlpy, et content; S Entropy, on chnges (entity):
Questions 1 13 cover material from Exam 1
 Questions 1 13 cover material from Exam 1 1. Which intermolecular forces are present in H Te(l)? A. dispersion only C. dispersion, dipole-dipole, and hydrogen bonding B. dispersion and dipole-dipole D.
Questions 1 13 cover material from Exam 1 1. Which intermolecular forces are present in H Te(l)? A. dispersion only C. dispersion, dipole-dipole, and hydrogen bonding B. dispersion and dipole-dipole D.
Chem 130 Third Exam. Total /100
 Nme Chem 130 Third Exm On the following pges you will find eight questions covering vries topics rnging from precipittion solubility, cid bse, nd oxidtion reduction rections to metl lignd complexes nd
Nme Chem 130 Third Exm On the following pges you will find eight questions covering vries topics rnging from precipittion solubility, cid bse, nd oxidtion reduction rections to metl lignd complexes nd
The Predom module. Predom calculates and plots isothermal 1-, 2- and 3-metal predominance area diagrams. Predom accesses only compound databases.
 Section 1 Section 2 The module clcultes nd plots isotherml 1-, 2- nd 3-metl predominnce re digrms. ccesses only compound dtbses. Tble of Contents Tble of Contents Opening the module Section 3 Stoichiometric
Section 1 Section 2 The module clcultes nd plots isotherml 1-, 2- nd 3-metl predominnce re digrms. ccesses only compound dtbses. Tble of Contents Tble of Contents Opening the module Section 3 Stoichiometric
dy ky, dt where proportionality constant k may be positive or negative
 Section 1.2 Autonomous DEs of the form 0 The DE y is mthemticl model for wide vriety of pplictions. Some of the pplictions re descried y sying the rte of chnge of y(t) is proportionl to the mount present.
Section 1.2 Autonomous DEs of the form 0 The DE y is mthemticl model for wide vriety of pplictions. Some of the pplictions re descried y sying the rte of chnge of y(t) is proportionl to the mount present.
Strong acids and bases. Strong acids and bases. Systematic Treatment of Equilibrium & Monoprotic Acid-base Equilibrium.
 Strong cids nd bses Systemtic Tretment of Equilibrium & Monoprotic cid-bse Equilibrium onc. (M) 0.0.00 -.00-5.00-8 p Strong cids nd bses onc. (M) p 0.0.0.00 -.0.00-5 5.0.00-8 8.0? We hve to consider utoprotolysis
Strong cids nd bses Systemtic Tretment of Equilibrium & Monoprotic cid-bse Equilibrium onc. (M) 0.0.00 -.00-5.00-8 p Strong cids nd bses onc. (M) p 0.0.0.00 -.0.00-5 5.0.00-8 8.0? We hve to consider utoprotolysis
CHEMGURU.ORG YOUTUBE ; CHEMGURU. Syllabus. Acids and Bases, ph, Common ion effect, Buffer solutions, Hydrolysis of salts and Solubility Product.
 Syllbus Acids nd Bses, ph, Common ion effect, Buffer solutions, Hydrolysis of slts nd Solubility Product. Acids nd Bses Here we discuss some importnt definitions of cids nd bses. Arrhenius Definition Arrhenius
Syllbus Acids nd Bses, ph, Common ion effect, Buffer solutions, Hydrolysis of slts nd Solubility Product. Acids nd Bses Here we discuss some importnt definitions of cids nd bses. Arrhenius Definition Arrhenius
First Semester Review Calculus BC
 First Semester Review lculus. Wht is the coordinte of the point of inflection on the grph of Multiple hoice: No lcultor y 3 3 5 4? 5 0 0 3 5 0. The grph of piecewise-liner function f, for 4, is shown below.
First Semester Review lculus. Wht is the coordinte of the point of inflection on the grph of Multiple hoice: No lcultor y 3 3 5 4? 5 0 0 3 5 0. The grph of piecewise-liner function f, for 4, is shown below.
FIRST EXAM. Answer any 4 of the following 5 questions. Please state any additional assumptions you made, and show all work.
 CEE 680 8 Mrch 018 FIRST EXAM Closed ook, one pge of notes llowed. Answer ny 4 of the following 5 questions. Plese stte ny dditionl ssumptions you mde, nd show ll work. Miscellneous Informtion: R = 1.987
CEE 680 8 Mrch 018 FIRST EXAM Closed ook, one pge of notes llowed. Answer ny 4 of the following 5 questions. Plese stte ny dditionl ssumptions you mde, nd show ll work. Miscellneous Informtion: R = 1.987
Solution W2009 NYB Final exam
 Solution W009 NYB Final exam Question Consider one liter of solution with [H SO 4 3.75 mol/l. Then : Mass of solution: 000 ml x.3 g/ml 30 g solution mass of H SO 4 : 3.75 mol /L x 98.078 g/mol 368 g mass
Solution W009 NYB Final exam Question Consider one liter of solution with [H SO 4 3.75 mol/l. Then : Mass of solution: 000 ml x.3 g/ml 30 g solution mass of H SO 4 : 3.75 mol /L x 98.078 g/mol 368 g mass
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this mteil useful? You cn help ou tem to keep this site up nd bing you even moe content conside donting vi the link on ou site. Still hving touble undestnding the mteil? Check out ou Tutoing pge to
Find this mteil useful? You cn help ou tem to keep this site up nd bing you even moe content conside donting vi the link on ou site. Still hving touble undestnding the mteil? Check out ou Tutoing pge to
For the entire exam, solutions are aqueous and T = 25 C unless stated otherwise. Questions 1 15 cover material from Exam 1.
 For the entire exam, solutions are aqueous and T = 25 C unless stated otherwise. Questions 1 15 cover material from Exam 1. 1. What state of matter is described as follows? On the molecular level, the
For the entire exam, solutions are aqueous and T = 25 C unless stated otherwise. Questions 1 15 cover material from Exam 1. 1. What state of matter is described as follows? On the molecular level, the
Math 211A Homework. Edward Burkard. = tan (2x + z)
 Mth A Homework Ewr Burkr Eercises 5-C Eercise 8 Show tht the utonomous system: 5 Plne Autonomous Systems = e sin 3y + sin cos + e z, y = sin ( + 3y, z = tn ( + z hs n unstble criticl point t = y = z =
Mth A Homework Ewr Burkr Eercises 5-C Eercise 8 Show tht the utonomous system: 5 Plne Autonomous Systems = e sin 3y + sin cos + e z, y = sin ( + 3y, z = tn ( + z hs n unstble criticl point t = y = z =
The Thermodynamics of Aqueous Electrolyte Solutions
 18 The Thermodynmics of Aqueous Electrolyte Solutions As discussed in Chpter 10, when slt is dissolved in wter or in other pproprite solvent, the molecules dissocite into ions. In queous solutions, strong
18 The Thermodynmics of Aqueous Electrolyte Solutions As discussed in Chpter 10, when slt is dissolved in wter or in other pproprite solvent, the molecules dissocite into ions. In queous solutions, strong
 Rel Gses 1. Gses (N, CO ) which don t obey gs lws or gs eqution P=RT t ll pressure nd tempertures re clled rel gses.. Rel gses obey gs lws t extremely low pressure nd high temperture. Rel gses devited
Rel Gses 1. Gses (N, CO ) which don t obey gs lws or gs eqution P=RT t ll pressure nd tempertures re clled rel gses.. Rel gses obey gs lws t extremely low pressure nd high temperture. Rel gses devited
CHAPTER 20: Second Law of Thermodynamics
 CHAER 0: Second Lw of hermodynmics Responses to Questions 3. kg of liquid iron will hve greter entropy, since it is less ordered thn solid iron nd its molecules hve more therml motion. In ddition, het
CHAER 0: Second Lw of hermodynmics Responses to Questions 3. kg of liquid iron will hve greter entropy, since it is less ordered thn solid iron nd its molecules hve more therml motion. In ddition, het
CHE 107 FINAL EXAMINATION December 10, 2012
 CHE 107 FINAL EXAMINATION December 10, 2012 University of Kentucky Department of Chemistry READ THESE DIRECTIONS CAREFULLY BEFORE STARTING THE EXAMINATION! It is extremely important that you fill in the
CHE 107 FINAL EXAMINATION December 10, 2012 University of Kentucky Department of Chemistry READ THESE DIRECTIONS CAREFULLY BEFORE STARTING THE EXAMINATION! It is extremely important that you fill in the
Ch 10 Practice Problems
 Ch 10 Practice Problems 1. Which of the following result(s) in an increase in the entropy of the system? I. (See diagram.) II. Br 2(g) Br 2(l) III. NaBr(s) Na + (aq) + Br (aq) IV. O 2(298 K) O 2(373 K)
Ch 10 Practice Problems 1. Which of the following result(s) in an increase in the entropy of the system? I. (See diagram.) II. Br 2(g) Br 2(l) III. NaBr(s) Na + (aq) + Br (aq) IV. O 2(298 K) O 2(373 K)
2. Which of the following liquids would have the highest viscosity at 25 C? A) CH 3 OCH 3 B) CH 2 Cl 2 C) C 2 H 5 OH D) CH 3 Br E) HOCH 2 CH 2 OH
 CHEF124 Mid Term Revision (Trimester 3, 2012/13) 1. Identify the dominant (strongest) type of intermolecular force present in (a) RbCl(s) ionic (b) NH 3 (l) - hydrogen bonding (c) Cl 2 (l) dispersion (d)
CHEF124 Mid Term Revision (Trimester 3, 2012/13) 1. Identify the dominant (strongest) type of intermolecular force present in (a) RbCl(s) ionic (b) NH 3 (l) - hydrogen bonding (c) Cl 2 (l) dispersion (d)
CHEMISTRY. 31 (b) The term acid rain was coined by Robert Augus 32 (c)
 CHEMISTRY 31 (b) The term cid rin ws coined by Robert Augus 32 (c) Initil At (4-3 moles equilibrium mole mole Hence, mole Hence, number of moles of t equilibrium =2-1=1 mole Number of moles of t equilibrium
CHEMISTRY 31 (b) The term cid rin ws coined by Robert Augus 32 (c) Initil At (4-3 moles equilibrium mole mole Hence, mole Hence, number of moles of t equilibrium =2-1=1 mole Number of moles of t equilibrium
Organic Acids - Carboxylic Acids
 Orgnic Acids - rboxylic Acids Orgnic cids - crboxylic cid functionl group rboxylic cids re redily deprotonted by bses such s NO eg 3 O O - + O - + O 3 O O Acid Bse onjugte Bse onjugte Acid This rection
Orgnic Acids - rboxylic Acids Orgnic cids - crboxylic cid functionl group rboxylic cids re redily deprotonted by bses such s NO eg 3 O O - + O - + O 3 O O Acid Bse onjugte Bse onjugte Acid This rection
First Law of Thermodynamics. Control Mass (Closed System) Conservation of Mass. Conservation of Energy
 First w of hermodynmics Reding Problems 3-3-7 3-0, 3-5, 3-05 5-5- 5-8, 5-5, 5-9, 5-37, 5-0, 5-, 5-63, 5-7, 5-8, 5-09 6-6-5 6-, 6-5, 6-60, 6-80, 6-9, 6-, 6-68, 6-73 Control Mss (Closed System) In this section
First w of hermodynmics Reding Problems 3-3-7 3-0, 3-5, 3-05 5-5- 5-8, 5-5, 5-9, 5-37, 5-0, 5-, 5-63, 5-7, 5-8, 5-09 6-6-5 6-, 6-5, 6-60, 6-80, 6-9, 6-, 6-68, 6-73 Control Mss (Closed System) In this section
Properties of Integrals, Indefinite Integrals. Goals: Definition of the Definite Integral Integral Calculations using Antiderivatives
 Block #6: Properties of Integrls, Indefinite Integrls Gols: Definition of the Definite Integrl Integrl Clcultions using Antiderivtives Properties of Integrls The Indefinite Integrl 1 Riemnn Sums - 1 Riemnn
Block #6: Properties of Integrls, Indefinite Integrls Gols: Definition of the Definite Integrl Integrl Clcultions using Antiderivtives Properties of Integrls The Indefinite Integrl 1 Riemnn Sums - 1 Riemnn
Chem 1B, Test Review #2
 1. The following kinetics data were obtained for the reaction: Expt.# 2NO(g) + Cl 2 (g) 2NOCl(g) [NO] 0 (mol/l) [Cl 2 ] 0 (mol/l) Initial Rate, (mol/l.s) 1 0.20 0.10 6.3 x 10 3 2 0.20 0.30 1.9 x 10 2 3
1. The following kinetics data were obtained for the reaction: Expt.# 2NO(g) + Cl 2 (g) 2NOCl(g) [NO] 0 (mol/l) [Cl 2 ] 0 (mol/l) Initial Rate, (mol/l.s) 1 0.20 0.10 6.3 x 10 3 2 0.20 0.30 1.9 x 10 2 3
ARITHMETIC OPERATIONS. The real numbers have the following properties: a b c ab ac
 REVIEW OF ALGEBRA Here we review the bsic rules nd procedures of lgebr tht you need to know in order to be successful in clculus. ARITHMETIC OPERATIONS The rel numbers hve the following properties: b b
REVIEW OF ALGEBRA Here we review the bsic rules nd procedures of lgebr tht you need to know in order to be successful in clculus. ARITHMETIC OPERATIONS The rel numbers hve the following properties: b b
CHM 2046 Test 2 Review: Chapter 12, Chapter 13, & Chapter 14
 Chapter 12: 1. In an 80.0 L home aquarium, the total pressure is 1 atm and the mole fraction of nitrogen is 0.78. Henry s law constant for N 2 in water at 25 is 6.1 x 10 4. What mass of nitrogen is dissolved
Chapter 12: 1. In an 80.0 L home aquarium, the total pressure is 1 atm and the mole fraction of nitrogen is 0.78. Henry s law constant for N 2 in water at 25 is 6.1 x 10 4. What mass of nitrogen is dissolved
CHEMISTRY 102 FALL 2010 FINAL EXAM FORM C Section 502 DR. KEENEY-KENNICUTT PART 1
 NAME (Block Print) CHEMISTRY 102 FALL 2010 FINAL EXAM FORM C Section 502 DR. KEENEY-KENNICUTT Directions: (1) Put your name on PART 1 and your name and signature on PART 2 of the exam where indicated.
NAME (Block Print) CHEMISTRY 102 FALL 2010 FINAL EXAM FORM C Section 502 DR. KEENEY-KENNICUTT Directions: (1) Put your name on PART 1 and your name and signature on PART 2 of the exam where indicated.
Acid-Base Equilibria
 Tdeusz Górecki Ionic Equiliri Acid-Bse Equiliri Brønsted-Lory: n cid is proton, se is. Acid Bse ( 3 PO 4, O), ( N 4 ) nd ( PO - 4 ) cn ll ehve s cids. Exmple: 4 N N3 Sustnces hich cn ehve oth s cids nd
Tdeusz Górecki Ionic Equiliri Acid-Bse Equiliri Brønsted-Lory: n cid is proton, se is. Acid Bse ( 3 PO 4, O), ( N 4 ) nd ( PO - 4 ) cn ll ehve s cids. Exmple: 4 N N3 Sustnces hich cn ehve oth s cids nd
CH 223 Sample Exam Exam II Name: Lab Section:
 Exam II Name: Lab Section: Part I: Multiple Choice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. Which of the following equations is the solubility
Exam II Name: Lab Section: Part I: Multiple Choice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. Which of the following equations is the solubility
SUMMER KNOWHOW STUDY AND LEARNING CENTRE
 SUMMER KNOWHOW STUDY AND LEARNING CENTRE Indices & Logrithms 2 Contents Indices.2 Frctionl Indices.4 Logrithms 6 Exponentil equtions. Simplifying Surds 13 Opertions on Surds..16 Scientific Nottion..18
SUMMER KNOWHOW STUDY AND LEARNING CENTRE Indices & Logrithms 2 Contents Indices.2 Frctionl Indices.4 Logrithms 6 Exponentil equtions. Simplifying Surds 13 Opertions on Surds..16 Scientific Nottion..18
5.111 Principles of Chemical Science
 MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Page 1 of 10 pages
MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Page 1 of 10 pages
temperature is known as ionic product of water. It is designated as K w. Value of K w
 Ionic product of ter The product of concentrtions of H nd OH ions in ter t prticulr temperture is knon s ionic product of ter. It is designted s K. H O H 1 OH ; H 57.3 kjm The vlue of [H ][OH ] K ; K[HO]
Ionic product of ter The product of concentrtions of H nd OH ions in ter t prticulr temperture is knon s ionic product of ter. It is designted s K. H O H 1 OH ; H 57.3 kjm The vlue of [H ][OH ] K ; K[HO]
CHM 2046 Final Exam Review: Chapters 11 18
 Chapter 11 1. Which of the following has the lowest boiling point? a. NH 3 b. CH 3 Cl c. NaCl d. CO 2 e. CH 3 CH 2 CH 2 CH 2 CH 3 2. Which of the following has the lowest vapor pressure? a. CH 3 F b. CH
Chapter 11 1. Which of the following has the lowest boiling point? a. NH 3 b. CH 3 Cl c. NaCl d. CO 2 e. CH 3 CH 2 CH 2 CH 2 CH 3 2. Which of the following has the lowest vapor pressure? a. CH 3 F b. CH
1. As the number of effective collisions between reacting particles increases, the rate of reaction (1) decreases (3) remains the same (2) increases
 1. As the number of effective collisions between reacting particles increases, the rate of reaction (1) decreases (3) remains the same (2) increases 2. The energy needed to start a chemical reaction is
1. As the number of effective collisions between reacting particles increases, the rate of reaction (1) decreases (3) remains the same (2) increases 2. The energy needed to start a chemical reaction is
CHEMpossible. Final Exam Review
 CHEMpossible Final Exam Review 1. Given the following pair of reactions and their equilibrium constants: 2NO 2 (g) 2NO (g) + O 2 (g) K c = 15.5 2NO (g) + Cl 2 (g) 2 NOCl (g) K c = 3.20 10-3 Calculate a
CHEMpossible Final Exam Review 1. Given the following pair of reactions and their equilibrium constants: 2NO 2 (g) 2NO (g) + O 2 (g) K c = 15.5 2NO (g) + Cl 2 (g) 2 NOCl (g) K c = 3.20 10-3 Calculate a
Physical Chemistry I for Biochemists Chem340. Lecture 38 (4/20/11) Yoshitaka Ishii. Announcement
 Physicl Chemistry I for Biochemists Chem34 Lecture 38 (4//11) Yoshitk Ishii Ch. 9.7 9.1 9.1 Announcement HW1 due dte is 4/7 (Wed) Exm 3 will be returned probbly this Fridy Finl Exm 5/4 (Wed) 1 3 pm Quiz
Physicl Chemistry I for Biochemists Chem34 Lecture 38 (4//11) Yoshitk Ishii Ch. 9.7 9.1 9.1 Announcement HW1 due dte is 4/7 (Wed) Exm 3 will be returned probbly this Fridy Finl Exm 5/4 (Wed) 1 3 pm Quiz
A proposed mechanism for the decomposition of hydrogen peroxide by iodide ion is: slow fast (D) H 2 O
 Chemistry 112, Spring 2007 Prof. Metz Exam 2 Practice Use the following information to answer questions 1 through 3 A proposed mechanism for the decomposition of hydrogen peroxide by iodide ion is: H 2
Chemistry 112, Spring 2007 Prof. Metz Exam 2 Practice Use the following information to answer questions 1 through 3 A proposed mechanism for the decomposition of hydrogen peroxide by iodide ion is: H 2
K P VERSUS K C PROPERTIES OF THE EQUILIBRIUM CONSTANT
 K P VERSUS K C 1. What are the units of K p and K c for each of the following? a) 2H 2 S(g) 2H 2 (g) + S 2 (g) b) 4NH 3 (g) + 3O 2 (g) 2N 2 (g) + 6H 2 O(g) 2. What are the units of K p and K c for each
K P VERSUS K C 1. What are the units of K p and K c for each of the following? a) 2H 2 S(g) 2H 2 (g) + S 2 (g) b) 4NH 3 (g) + 3O 2 (g) 2N 2 (g) + 6H 2 O(g) 2. What are the units of K p and K c for each
Second Law of Thermodynamics
 Second Law of Thermodynamics First Law: the total energy of the universe is a constant Second Law: The entropy of the universe increases in a spontaneous process, and remains unchanged in a process at
Second Law of Thermodynamics First Law: the total energy of the universe is a constant Second Law: The entropy of the universe increases in a spontaneous process, and remains unchanged in a process at
1 This question is about mean bond enthalpies and their use in the calculation of enthalpy changes.
 1 This question is out men ond enthlpies nd their use in the lultion of enthlpy hnges. Define men ond enthlpy s pplied to hlorine. Explin why the enthlpy of tomistion of hlorine is extly hlf the men ond
1 This question is out men ond enthlpies nd their use in the lultion of enthlpy hnges. Define men ond enthlpy s pplied to hlorine. Explin why the enthlpy of tomistion of hlorine is extly hlf the men ond
Part I: Basic Concepts of Thermodynamics
 Prt I: Bsic Concepts o Thermodynmics Lecture 4: Kinetic Theory o Gses Kinetic Theory or rel gses 4-1 Kinetic Theory or rel gses Recll tht or rel gses: (i The volume occupied by the molecules under ordinry
Prt I: Bsic Concepts o Thermodynmics Lecture 4: Kinetic Theory o Gses Kinetic Theory or rel gses 4-1 Kinetic Theory or rel gses Recll tht or rel gses: (i The volume occupied by the molecules under ordinry
CH 221 Sample Exam Exam II Name: Lab Section:
 Exam II Name: Lab Section: Part I: Multiple Choice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. When methanol undergoes complete combustion,
Exam II Name: Lab Section: Part I: Multiple Choice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. When methanol undergoes complete combustion,
13.4 Work done by Constant Forces
 13.4 Work done by Constnt Forces We will begin our discussion of the concept of work by nlyzing the motion of n object in one dimension cted on by constnt forces. Let s consider the following exmple: push
13.4 Work done by Constnt Forces We will begin our discussion of the concept of work by nlyzing the motion of n object in one dimension cted on by constnt forces. Let s consider the following exmple: push
Narayana IIT Academy
 INDIA Sec: Sr. IIT_IZ Jee-Advnced Dte: --7 Time: 09:00 AM to :00 Noon 0_P Model M.Mrks: 0 KEY SHEET CHEMISTRY C D 3 D B 5 A 6 D 7 B 8 AC 9 BC 0 ABD ABD A 3 C D 5 B 6 B 7 9 8 9 0 7 8 3 3 6 PHYSICS B 5 D
INDIA Sec: Sr. IIT_IZ Jee-Advnced Dte: --7 Time: 09:00 AM to :00 Noon 0_P Model M.Mrks: 0 KEY SHEET CHEMISTRY C D 3 D B 5 A 6 D 7 B 8 AC 9 BC 0 ABD ABD A 3 C D 5 B 6 B 7 9 8 9 0 7 8 3 3 6 PHYSICS B 5 D
Quiz B3: Le Chatelier s Principle Block:
 Quiz B3: Le Chatelier s Principle Name: Block: 1. Consider the following reaction: 2SO2(g) + O2(g) 2SO3(g) H = -197 kj/mol Which of the following will not shift the equilibrium to the right? A. Adding
Quiz B3: Le Chatelier s Principle Name: Block: 1. Consider the following reaction: 2SO2(g) + O2(g) 2SO3(g) H = -197 kj/mol Which of the following will not shift the equilibrium to the right? A. Adding
Lecture 6: Diffusion and Reaction kinetics
 Lecture 6: Diffusion nd Rection kinetics 1-1-1 Lecture pln: diffusion thermodynmic forces evolution of concentrtion distribution rection rtes nd methods to determine them rection mechnism in terms of the
Lecture 6: Diffusion nd Rection kinetics 1-1-1 Lecture pln: diffusion thermodynmic forces evolution of concentrtion distribution rection rtes nd methods to determine them rection mechnism in terms of the
DIRECT CURRENT CIRCUITS
 DRECT CURRENT CUTS ELECTRC POWER Consider the circuit shown in the Figure where bttery is connected to resistor R. A positive chrge dq will gin potentil energy s it moves from point to point b through
DRECT CURRENT CUTS ELECTRC POWER Consider the circuit shown in the Figure where bttery is connected to resistor R. A positive chrge dq will gin potentil energy s it moves from point to point b through
Problem 22: Buffer solutions 1. The equilibrium, which governs the concentration of H + within the solution is HCOOH! HCOO + H + + Hence K
 Problem : Buffer solutions. The equilibrium, hich governs the concentrtion of H ithin the solution is HCOOH! HCOO H [HCOO ] 4 Hence. [HCOOH] nd since [HCOOH] 0.00 M nd [HCOO ] 0.50 M -4 0.00 4..8 M 0.50
Problem : Buffer solutions. The equilibrium, hich governs the concentrtion of H ithin the solution is HCOOH! HCOO H [HCOO ] 4 Hence. [HCOOH] nd since [HCOOH] 0.00 M nd [HCOO ] 0.50 M -4 0.00 4..8 M 0.50
(2). Cu(NO 3 ) HCl CuCl HNO 3 all products soluble, no ppt
 CHEM 112 Solubility and Precipitation Rexn 25.0mL 0.010M HCl 25.0mL 0.10M NaOH 50.0mL 0.0010M 25.0mL 0.050M 50.0mL 0.0010M AgNO Fe(NO ) Cu(NO ) 2 (1). Fe(NO ) + HCl FeCl + HNO all products soluble, no
CHEM 112 Solubility and Precipitation Rexn 25.0mL 0.010M HCl 25.0mL 0.10M NaOH 50.0mL 0.0010M 25.0mL 0.050M 50.0mL 0.0010M AgNO Fe(NO ) Cu(NO ) 2 (1). Fe(NO ) + HCl FeCl + HNO all products soluble, no
MAT137 Calculus! Lecture 20
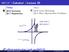 officil website http://uoft.me/mat137 MAT137 Clculus! Lecture 20 Tody: 4.6 Concvity 4.7 Asypmtotes Net: 4.8 Curve Sketching 4.5 More Optimiztion Problems MVT Applictions Emple 1 Let f () = 3 27 20. 1 Find
officil website http://uoft.me/mat137 MAT137 Clculus! Lecture 20 Tody: 4.6 Concvity 4.7 Asypmtotes Net: 4.8 Curve Sketching 4.5 More Optimiztion Problems MVT Applictions Emple 1 Let f () = 3 27 20. 1 Find
Accelerated Chemistry Semester 2 Review Sheet
 Accelerated Chemistry Semester 2 Review Sheet The semester test will be given in two parts. The first part is a performance assessment and will be given the day before the semester test. This will include
Accelerated Chemistry Semester 2 Review Sheet The semester test will be given in two parts. The first part is a performance assessment and will be given the day before the semester test. This will include
FACULTY OF SCIENCE MID-TERM EXAMINATION 2 MARCH 18, :30 TO 8:30 PM CHEMISTRY 120 GENERAL CHEMISTRY
 FACULTY OF SCIENCE MID-TERM EXAMINATION 2 MARCH 18, 2011. 6:30 TO 8:30 PM CHEMISTRY 120 GENERAL CHEMISTRY Examiners: Prof. B. Siwick Prof. A. Mittermaier Dr. A. Fenster Name: Associate Examiner: A. Fenster
FACULTY OF SCIENCE MID-TERM EXAMINATION 2 MARCH 18, 2011. 6:30 TO 8:30 PM CHEMISTRY 120 GENERAL CHEMISTRY Examiners: Prof. B. Siwick Prof. A. Mittermaier Dr. A. Fenster Name: Associate Examiner: A. Fenster
The Factors that Determine the Equilibrium State
 The Factors that Determine the Equilibrium State The equilibrium state (or the ratio of products to reactants) is determined by two factors: 1. Energy Systems tend to move toward a state of minimum potential
The Factors that Determine the Equilibrium State The equilibrium state (or the ratio of products to reactants) is determined by two factors: 1. Energy Systems tend to move toward a state of minimum potential
Chem 1412 Final Exam. Student:
 Chem 1412 Final Exam Student: 1. The radiochemist, Will I. Glow, studied thorium-232 and found that 2.82 10-7 moles emitted 8.42 10 6 α particles in one year. What is the decay constant for thorium-232?
Chem 1412 Final Exam Student: 1. The radiochemist, Will I. Glow, studied thorium-232 and found that 2.82 10-7 moles emitted 8.42 10 6 α particles in one year. What is the decay constant for thorium-232?
CHM Physical Chemistry I Chapter 1 - Supplementary Material
 CHM 3410 - Physicl Chemistry I Chpter 1 - Supplementry Mteril For review of some bsic concepts in mth, see Atkins "Mthemticl Bckground 1 (pp 59-6), nd "Mthemticl Bckground " (pp 109-111). 1. Derivtion
CHM 3410 - Physicl Chemistry I Chpter 1 - Supplementry Mteril For review of some bsic concepts in mth, see Atkins "Mthemticl Bckground 1 (pp 59-6), nd "Mthemticl Bckground " (pp 109-111). 1. Derivtion
200 points 5 Problems on 4 Pages and 20 Multiple Choice/Short Answer Questions on 5 pages 1 hour, 48 minutes
 PHYSICS 132 Smple Finl 200 points 5 Problems on 4 Pges nd 20 Multiple Choice/Short Answer Questions on 5 pges 1 hour, 48 minutes Student Nme: Recittion Instructor (circle one): nme1 nme2 nme3 nme4 Write
PHYSICS 132 Smple Finl 200 points 5 Problems on 4 Pges nd 20 Multiple Choice/Short Answer Questions on 5 pges 1 hour, 48 minutes Student Nme: Recittion Instructor (circle one): nme1 nme2 nme3 nme4 Write
KWANTLEN UNIVERSITY COLLEGE DEPARTMENT OF CHEMISTRY
 KWANTLEN UNIVERSITY COLLEGE DEPARTMENT OF CHEMISTRY NAME: CHEM. 1210 FINAL EXAMINATION December 13, 2001 Time: 3 hours INSTRUCTIONS: 1. Read all questions thoroughly and answer each question completely.
KWANTLEN UNIVERSITY COLLEGE DEPARTMENT OF CHEMISTRY NAME: CHEM. 1210 FINAL EXAMINATION December 13, 2001 Time: 3 hours INSTRUCTIONS: 1. Read all questions thoroughly and answer each question completely.
Kinematics equations, some numbers
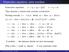 Kinemtics equtions, some numbers Kinemtics equtions: x = x 0 + v 0 t + 1 2 t2, v = v 0 + t. They describe motion with constnt ccelertion. Brking exmple, = 1m/s. Initil: x 0 = 10m, v 0 = 10m/s. x(t=1s)
Kinemtics equtions, some numbers Kinemtics equtions: x = x 0 + v 0 t + 1 2 t2, v = v 0 + t. They describe motion with constnt ccelertion. Brking exmple, = 1m/s. Initil: x 0 = 10m, v 0 = 10m/s. x(t=1s)
Basic Derivative Properties
 Bsic Derivtive Properties Let s strt this section by remining ourselves tht the erivtive is the slope of function Wht is the slope of constnt function? c FACT 2 Let f () =c, where c is constnt Then f 0
Bsic Derivtive Properties Let s strt this section by remining ourselves tht the erivtive is the slope of function Wht is the slope of constnt function? c FACT 2 Let f () =c, where c is constnt Then f 0
x dx does exist, what does the answer look like? What does the answer to
 Review Guie or MAT Finl Em Prt II. Mony Decemer th 8:.m. 9:5.m. (or the 8:3.m. clss) :.m. :5.m. (or the :3.m. clss) Prt is worth 5% o your Finl Em gre. NO CALCULATORS re llowe on this portion o the Finl
Review Guie or MAT Finl Em Prt II. Mony Decemer th 8:.m. 9:5.m. (or the 8:3.m. clss) :.m. :5.m. (or the :3.m. clss) Prt is worth 5% o your Finl Em gre. NO CALCULATORS re llowe on this portion o the Finl
MODIFICATION OF VANT HOFF PARAMETER FOR CONCENTRATE SALT SOLUTION
 VOL. 13, NO. 9, MAY 018 ISSN 18196608 ARN Journl of Engineering nd Applied Sciences 006018 Asin Reserch ulishing Network (ARN). All rights reserved. www.rpnjournls.com MODIFICATION OF VANT HOFF ARAMETER
VOL. 13, NO. 9, MAY 018 ISSN 18196608 ARN Journl of Engineering nd Applied Sciences 006018 Asin Reserch ulishing Network (ARN). All rights reserved. www.rpnjournls.com MODIFICATION OF VANT HOFF ARAMETER
Chem 116 POGIL Worksheet - Week 12 - Solutions Second & Third Laws of Thermodynamics Balancing Redox Equations
 Chem 116 POGIL Worksheet - Week 12 - Solutions Second & Third Laws of Thermodynamics Balancing Redox Equations Key Questions 1. Does the entropy of the system increase or decrease for the following changes?
Chem 116 POGIL Worksheet - Week 12 - Solutions Second & Third Laws of Thermodynamics Balancing Redox Equations Key Questions 1. Does the entropy of the system increase or decrease for the following changes?
f a L Most reasonable functions are continuous, as seen in the following theorem:
 Limits Suppose f : R R. To sy lim f(x) = L x mens tht s x gets closer n closer to, then f(x) gets closer n closer to L. This suggests tht the grph of f looks like one of the following three pictures: f
Limits Suppose f : R R. To sy lim f(x) = L x mens tht s x gets closer n closer to, then f(x) gets closer n closer to L. This suggests tht the grph of f looks like one of the following three pictures: f
Lecture 5. Selected aspects of thermodynamics for adsorption, diffusion and desorption
 Physics 986b Lecture 5 Selected spects of thermodynmics for dsorption, diffusion nd desorption Physisorption Chemisorption Surfce Bonding Mechnisms of dsorption/diffusion/desorption References: 1) Zngwill,
Physics 986b Lecture 5 Selected spects of thermodynmics for dsorption, diffusion nd desorption Physisorption Chemisorption Surfce Bonding Mechnisms of dsorption/diffusion/desorption References: 1) Zngwill,
MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, :30PM 8:30PM VERSION NUMBER: 1
 MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, 2009 6:30PM 8:30PM VERSION NUMBER: 1 Instructions: BEFORE YOU BEGIN: Enter your student number and name on the computer
MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, 2009 6:30PM 8:30PM VERSION NUMBER: 1 Instructions: BEFORE YOU BEGIN: Enter your student number and name on the computer
CHEMICAL EQUILIBRIUM Chapter 13
 1 CHEMICAL EQUILIBRIUM Chapter 13 Pb 2+ (aq) + 2 Cl (aq) PbCl 2 (s) 1 Objectives Briefly review what we know of equilibrium Define the Equilibrium Constant (K eq ) and Reaction Quotient (Q) Determining
1 CHEMICAL EQUILIBRIUM Chapter 13 Pb 2+ (aq) + 2 Cl (aq) PbCl 2 (s) 1 Objectives Briefly review what we know of equilibrium Define the Equilibrium Constant (K eq ) and Reaction Quotient (Q) Determining
Conservation Law. Chapter Goal. 5.2 Theory
 Chpter 5 Conservtion Lw 5.1 Gol Our long term gol is to understnd how mny mthemticl models re derived. We study how certin quntity chnges with time in given region (sptil domin). We first derive the very
Chpter 5 Conservtion Lw 5.1 Gol Our long term gol is to understnd how mny mthemticl models re derived. We study how certin quntity chnges with time in given region (sptil domin). We first derive the very
